Demineralization Process
The process of demineralizing water or solutions by ion exchange consists of the conversion of salts to their corresponding acids by hydrogen cation exchangers and the removal of these acids by anion exchangers. salts dissolved in water dissociate into positively charged ions (cations) and negatively charged ions (anions), which allow the solution to conduct electricity (electrolytes). Likewise ion exchangers contain positively charged cations and negatively charged anions in a condition of electro-neutrality. The exchangers differ from solutions in that only one of the two ionic species is mobile (exchangeable).
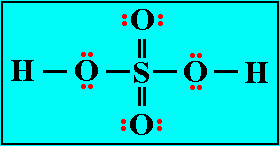
For example a typical sulfuric acid cation exchanger has an immobile ion exchange site consisting of SO31- radicals to which are attached mobile cations, such as H+ or Na+, that may be exchanged in an ion exchange reaction. An anion exchanger similarly has immobile cationic sites to which are attached mobile hydroxide anions. |
 |
When ion exchange occurs, the cations or anions in the solution are interchanged for those in the exchanger, but both the solution and the exchanger remain in a condition of electro-neutrality. In the case of the cation exchanger with calcium cations which has 2 positive charges (Ca2+), when calcium leaves, the water must replace two hydrogen cations in the exchanger which have a single positive charge (H+).
Click here to continue to demineralization process to prepare water for use in the semiconductor industry .