A distillation column is a piece of equipment that seperates two or more components (chemicals) from each other based on differences in boiling points. The details of how this works and how to calculate what is going on inside the column will be learned in a class called Mass Transfer.
A basic schematic of a distillation column looks like:
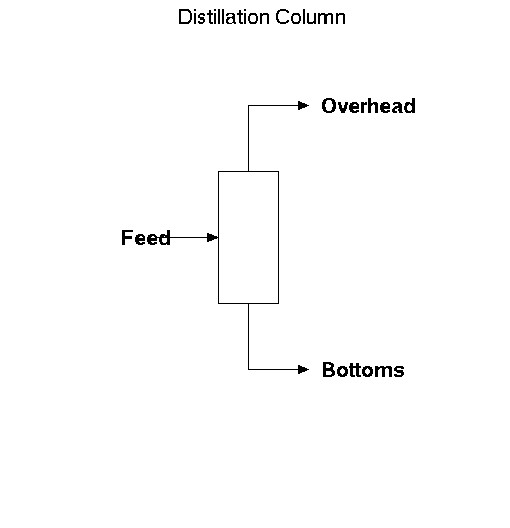
The feed comes into the column, while the overhead and bottoms flow out of the column. The overhead streams contains the lighter chemicals. When we say that, we mean that it contains the chemicals that boil at a lower temperature or have higher volatilities (are more likely to be in the gas phase). The bottoms stream has more of the heavier, or higher boiling point, components.
Alternate names:
distillation column = fractionator, fractionating column, still, column, staged seperator, thermal seperator.
feed = inlet, feed stock, input.
overhead = distillate, vapor product, light key product, condensate.
bottoms = distilland, liquid product, heavy key product.
Variations on distillation column arrangements:
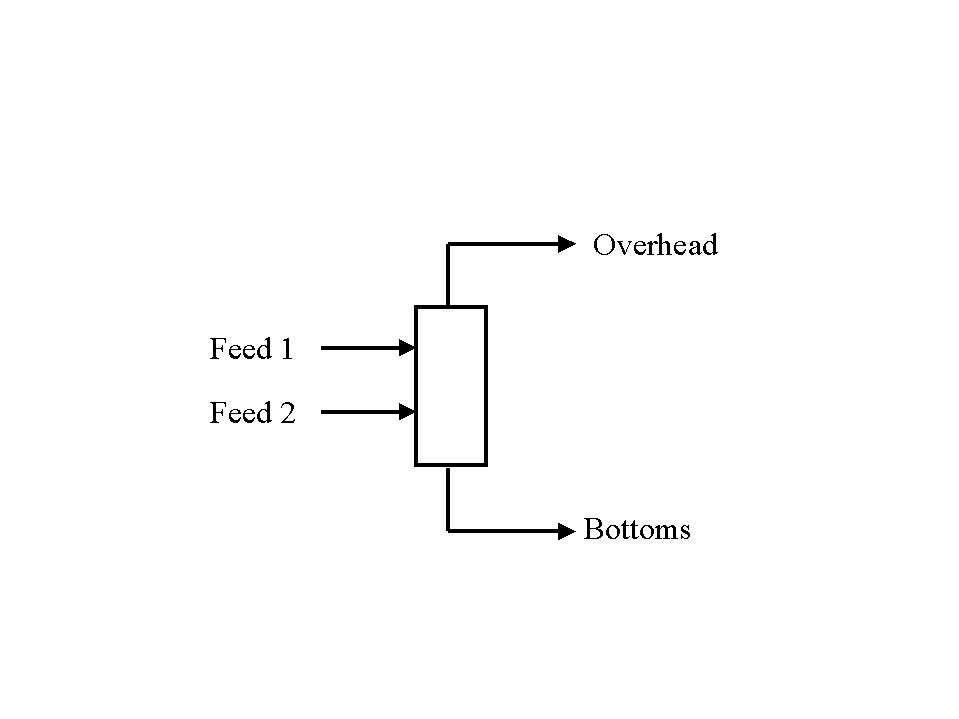
More than one feed stream: