
An example of Fick's Law
Example 1
Fick's Law states: j = DAB DC / DX
In this case, DC is equal to the change in concentration, Csat - Cbulk
DX is equal to the distance between Csat and Cbulk which is l.
Thus we find: j = 1.14 * 10-4 (1.77 - 0.538) / 0.00001 = 14
Now, j is measured in moles caffeine / second * meter squared. Thus if we multiply by the surface area of the coffee ground we would find the the amount of caffeine produced from the coffee grounds per second. The amount of caffeine produced per time is therefore directly related to the surface area of the coffee ground. If we double the size of the surface area of the coffee ground, we halve the time it takes to brew. Grinding the the coffee increases the surface area of the coffee tremendously. Can you imagine the time it would take to brew coffee from whole beans?
If we take a coffee bean to have roughly 1 cm2 surface area, and there to be about 200 coffee beans used to brew a pot of coffee, we find that they have an initial surface area of .02 m2. Coarse powders of the approximate size of a coffee ground tend to have 50 meters m2 per gram. That's about 800 m2 surface area of coffee per pot, an increase of 40,000%! If it takes only one second of contact between the water and the contact between the water and the ground coffee, it would take 40,000 seconds to brew a pot of coffee -- that's over 11 hours! The entire pot of coffee would have to be just below boiling and within .00001 meters of a coffee bean for almost a half day. That's a lot of energy and a lot of coffee beans.
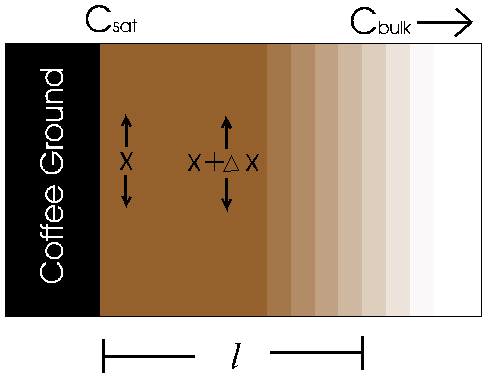
In the figure above, we see a diagram of the caffeine concentration very near to the surface of a coffee ground. As you can see, the concentration decreases from Csat to Cbulk as you move across the distance l from the surface of the ground. We can assume the concentration of caffeine to be the same at all points of an equal distance from the surface, forming layers of equal concentration. We are also going to assume that the layers are at steady state. That is, the concentrations of an individual layer does not change over time. We will now make use of the basic equation of chemical engineering:
In - Out + Generation - Consumption = Accumulation
This equation can be interpreted in words: Caffeine diffusing In to a layer minus caffeine Diffusing out of a layer plus caffeine Generated in a layer minus caffeine Consumed in a layer is equal to the rate at which caffeine in a layer Accumulates. Now there are no chemical reactions either producing or consuming caffeine in the brewing of coffee. Thus both the Generation and Consumption terms are both zero. We are left with:
In - Out = Accumulation
We will apply this equation to a single layer of of coffee beginning a distance X from the surface of the coffee and ending at distance X + DX. The volume of this layer is equal to its thickness, DX times the surface area of the coffee ground, A. By multiplying the volume of the layer by the concentration of caffeine in the layer, we can find the total amount of caffeine in the layer, DX*A*C. Accumulation is equal to the change in the total caffeine over time, denoted:
d(DXAC)/dt = Accumulation
In this type of notation the term next to the 'd' in the numerator is changing with respect to the term next to the 'd' in the denominator. In this case total caffeine, rXAC is changing with respect to time, t. Because the volume of the layer does not change as time passes, we may factor it out and write:
DXA * dC/dt
The In term represents the caffeine passing into the layer at distance X from the surface of the coffee ground. Similarly the Out term is the caffeine passing out of the layer at a distance X + DX from the surface of the ground. Our equation can now be written as:
jA|X + jA|X+DX = DXA * dC/dt
Dividing both sides by A, we get:
j|X + j|X+DX = DX * dC/dt
We also stated above that the concentration of caffeine in an individual layer does not change with time. Therefore there is neither caffeine accumulating or dissipating thus the Accumulation term is zero as well. We are now left with :
j|X + j|X+DX = 0
To evaluate this, we will divide the equation by DX, and evaluate the equation as DX approaches zero. This is a procedure known as taking a derivative. In this case it represents letting our layer's thickness become infinitely small and evaluating amount of caffeine in this layer. This is done for an infinite number of layers and then added together so that we can find the total amount of caffeine in the layer l. The equation now looks like:
dj/dx = 0
We showed in the first example that J = DAB dC/dX. Therefore:
d(DAB dC/dX)/dX = 0
We now perform an operation called integration. This is a way of reversing the derivatives in the equation above, much like multiplying will cancel out a division. Because there are two derivatives in the equation, we will need to integrate twice. Each integration will produce a unknown constant which we will have to solve for in the next step. We will represent these unknowns with the variables P and Q. We will first distribute the dX to get:
d(DAB dC/dX) = dX integration yields:
DAB dC/dX = X + P dividing by DAB and 'multiplying' dX yields:
dC/dX = (X / DAB + P / DAB)dX integrating again yields:
C = X2 / (2 * DAB) + P * X / DAB + Q
We are now left with an equation for concentration in terms of distance X and two constants P and Q. To solve for these constants we will apply what is known as boundary conditions.