The reaction between propane and hydrogen chloride to form propyl chloride and hydrogen is carried out in a continuous reactor. The product stream is analyzed and found to contain 27.45 mole% C3H7Cl, 27.45 mole % H2 and 14.6% HCl. The feed to the reactor contains only propane and hydrogen chloride. Calculate the fractional conversion of the limiting reactant and the percentage by which the other reactant is in excess. If the molar flow rate of the feed stream is 290 mol/s, what is the extent of reaction? (Give its numerical value and its units.)
Explanation:
A manometer is used to find out information about pressure. If you think back to the manometer equations we learned how to derive:
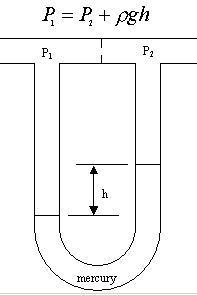
the variables that are involved are pressures (P's), densities (ρ), gravity (g), and heights (h's). We don't have any of these variables in our problem. Well, we could look up densities, but we don't have any heights related to manometers, or any pressures at all. So we don't need a manometer equation in this problem.
