Pressure Topics
Upon studying this section, you should be familiar with the following:
- Why we can express the units of pressure as the length of a fluid.
- The interrelation between gauge pressure to absolute pressure.
- Solving manometer and other static pressure problems.
Note on Pressure Units
| Explanation:
From physics we learn that Pressure = Force/Area, or P = F/A, and F/A can also be written as distance*density*gravity.So, Pliquid = distance * gravity * density now, when we recognize that gravity is constant at sea level, and density is relatively constant for any liquid, then we can see that, Pliquid = distance * gravity * density = distance * constant, This shows that pressure is a direct function only of the distance (vertical distance) of a liquid between points, so because the gravity and the density of liquid are constant, pressure can be expressed with units of liquid height, such as ft H2O, or mm Hg. |
Gauge Pressure
| Explanation:
Gauge pressure, or Pgauge, doesn't take into account the atmospheric pressure in its readings. In chemical engineering, unless otherwise stated, we want to use absolute pressure in calculations. Converting from Pgauge to Pabsolute is done using the following formula: Pabs = Pgauge + Patm note: Let me re-emphasize, when pressure is initially given in gauge,
alway's first convert Pgauge to Pabsolute before
any other calculations.
Example 3.a.1: Perform the following pressure conversions. Assume atmospheric pressure is 1 atm. Unless other wise stated, the pressures are given in absolute.
|
Hydrostatic Pressure
| Explanation:
The purpose of this section is to introduce the interrelation that relates the pressure exerted by a fluid or solid to its density. For some fluid or solid, designated i, at height h above a referrence point, we have the following interrelation. Pi = ρigh 
Pwater = ρwatergh Now, just for fun, I pose the following question. Is the pressure exerted on the bottom of the flask completely given by the water in the flask? That is, is there anything else exerting pressure on the bottom of the flask? The answer is yes, there is air exerting pressure on top of the water. So, while we have just written an expression for the pressure exerted on the bottom of the cup due to water, if we want to find to total pressure exerted on the bottom of the cup, we must account for the atmospheric pressure being exerted on the water. So our expression for the total pressure exerted on the bottom of the cup would include the atmospheric pressure: Ptotal = ρwatergh + Patm
This pressure is called the hydrostatic pressure. I wanted to formally introduce this interrelation here, and a practical application of this interrelation is given in the next section. |
Manometers and Static Pressure Problems
| Explanation:
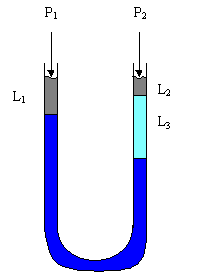
Typical applications to the interrelations given above are in manometer problems. A typical manometer could be exposed to different pressures at it's openings and is filled with one or more different fluids. A typical manometer problem could ask you to find the pressure drop across the two openings. Here, given the following diagram, we derive an expression for the pressure drop.
Here, LHS and RHS represent the left and right hand side of the manometer. We begin with writing the pressure representation of each component, then we gradually simplify the expression to get it into a reduced form. Ptop,LHS + Pair,LHS + Pw,LHS = Ptop,RHS + Pair,RHS + PMe,RHS + Pw,RHS or Ptop,LHS + ρairghair,LHS + ρwghw,LHS = Ptop,RHS + ρairghair,RHS + ρMeghMe,RHS + ρwghw,RHS
or, with heights known, and LT representing the total length of the manometer, we get, Ptop,LHS + ρairgL1 + ρwg(LT-L1) = Ptop,RHS + ρairgL2 + ρMegL3 + ρwg(LT - L2 - L3)
now, we can rearrange the equation, with the total pressure drop written as DP Ptop,LHS - Ptop,RHS = ρairgL2 - ρairgL1 + ρwg(LT - L2 - L3) - ρwg(LT-L1) + ρMegL3
or, DP = ρairg(L2 - L1) + ρwg(L1 - L2 - L3) + ρMegL3
This is our expression, given or looking up the quantities on the right side of the equation allows for a determination of the pressure drop. Example 3.a.2: The great Boston molasses flood occurred on January 15, 1919. In it,
2.3 million gallons of crude molasses flowed from a 30-foot high storage
tank that ruptured, killing 21 people and injuring 150. The estimated specific
gravity of crude molasses is 1.4 (so the density of the molasses was 1.4
g/ml). What was the mass of molasses in the tank?
Example 3.a.3: What was the pressure at the bottom of the molasses tank prior to the tank rupture?
Assume that the tank was vented to the atmosphere.
|

The University of Arizona. All copyrights © reserved.