Explanation:
Ok, since being introduced to multicomponent equilibrium, we have discussed how to deal with gas mixtures (Dalton's law of partial pressures), vapor-liquid systems (Raoult's law and phase diagrams), and colligative effects of a solute in a solution. Now for our last discussion involving phase equilibrium, we have liquid solutions. That is, systems like liquid oil, with butanol, and water. At some temperature and pressure, if we have a mixture of the three, we can get a single, liquid phase. Let's say we add some more oil and suddenly get two distinct liquid phases, with one mostly water, and the other mostly oil. The butanol is dispersered between the two phases. How much of each substance (water, oil, and butanol) do we have in the liquid phase that is mostly oil (the oil rich phase)? Also, what are the mole fractions of these same compounds in the water rich phase? Well, this is a relatively simple series of questions to answer with a ternary phase diagram. This type of diagram is used for 3 component systems that either make up one liquid phase or separtate into 2 liquid phases. The diagram gives the amount of component in the respective phase.
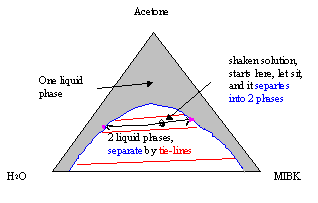
To follow the discussion here, just note that you can read off all three compositions for any point on a diagram. When we mix up a solution, shake it and end up outside the (blue curve), only one phase will appear. This means that we can only see a single uniform mixture that is the same everywhere. Things become interesting when we mix up a combination that is under the blue curve. After we initially finish the shaking, it looks like there is a single liquie phase like when we are above the blue line. However, after a few minutes, you'll begin to notice that two separate liquids are in the container. A good example of this happening is when you mix oil and vinegar to make a salad dressing. You've probably seen homemade dressing separate just like we're describing here. When the solution is allowed to sit undisturbed for a while, it settles into two phases. When we know the initial ("shaken") concentration (the black dot on the phase diagram), we can find the concentration of the two separted liquid phases by following the tie lines (red lines) out to the two phase boundary (the blue arc). At the two phase boundary on either end of the tie-line, we can read off the two distinct phase compositions for the oil-rich and the water-rich phases.
You can use the ternary phase diagram in your text-book to solve the following problems.
Example 1:
If two streams are mixed together to give concentrations of 35% H2O and 50% Acetone, what is the initial concentration? If the stream is allowed to settle, what is the equilibrium concentrations of each phase? Does it form separate phases? If it doesn't, and we add MIBK, what is the concentration of MIBK when it begins to separte into two phases? Repeat the calculations and answer the questions for a initially shaken concentration of 45% H2O and 40% acetone.
Example 2:
One thousand kilograms of a 30.0% by weight solution of acetone in water and a second stream of pure MIBK is fed to a mixer. The mixture is then fed to a settler where two phases form and are withdrawn separately at 25°C. How much MIBK must be fed to the process to reduce the acetone concentration in the water-rich phase to 5 wt%, assuming that the fluids remain in the settler long enough for equilibrium to be achieved, of course?
Example 3:
An aqueous acetone solutions is fed at a rate of 32.0 lbm/h to a stirred tank. A stream of pure MIBK is also fed to the tank, and the resulting mixture is sent to a settler operating at 25°C. One of the phases formed has a flowrate of 41.0 lbm/h and contains 70 wt% MIBK. Determine the flow rate and composition of the second product stream and the rate at which MIBK is fed to the unit.
