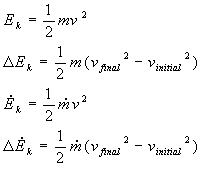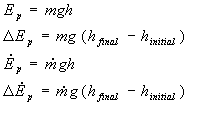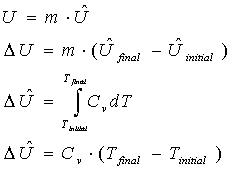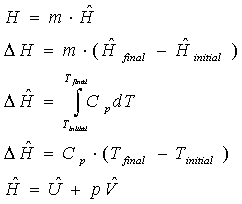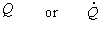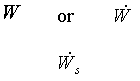|
Explanation:
There are really three types of energy that we'll deal with often in chemical engineering: Kinetic Energy, Ek, Potential Energy, Ep, and Internal Energy, U. Closely related to Internal Energy is Enthalpy, H. Now, lets introduce the definitions, mathematical descriptions, and the concepts for each type of energy.
Kinetic Energy
Definition: Energy due to motion
Relation:
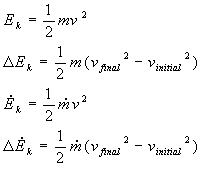 Where m is for mass, v is for velocity, and the dot signifies a rate.
Examples: A moving car, a gas molecule, and a flowing stream all have kinetic energy. A parked car, a molecule in a solid lattice, and a stationary glass of water are all systems that do not possess kinetic energy (because they do not have a non-zero velocity).
Note: If the system is not accelerating or decelerating (changing velocity), DEK = 0. That is, the system has kinetic energy, but that energy doesn't change as you move between two point unless the system has undergone a change in velocity: the change in kinetic energy is zero.
In the bulk of energy problems that you will deal with, that is, those in which there is a chemical reaction, a change of state, or substantial temperature change, the change in kinetic energy is very small (DEk = 0).
Where m is for mass, v is for velocity, and the dot signifies a rate.
Examples: A moving car, a gas molecule, and a flowing stream all have kinetic energy. A parked car, a molecule in a solid lattice, and a stationary glass of water are all systems that do not possess kinetic energy (because they do not have a non-zero velocity).
Note: If the system is not accelerating or decelerating (changing velocity), DEK = 0. That is, the system has kinetic energy, but that energy doesn't change as you move between two point unless the system has undergone a change in velocity: the change in kinetic energy is zero.
In the bulk of energy problems that you will deal with, that is, those in which there is a chemical reaction, a change of state, or substantial temperature change, the change in kinetic energy is very small (DEk = 0).
Potential Energy
Definition: Energy due to position
Relation:
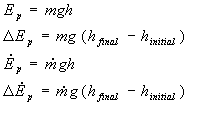 Where m is for mass, g is gravity, h is a height, and the dot signifies a rate
Where m is for mass, g is gravity, h is a height, and the dot signifies a rate
Examples: A bungee jumper standing on the edge of a cliff, a book resting on a table, a jacket hung in a closet, or anything that is above the ground possesses potential energy due to gravity. So, someone standing on the ground, and shoes that rest on the ground are both systems that possess no potential energy due to gravity.
Note: If the system neither rises nor falls, the change in potential energy is zero (DEp = 0). Additionally, rarely does the system rise or fall substantially in the energy balance problems that you will perform for chemical engineering problems in this class, so you will find that the potential energy change is often negligable and set to zero. Finally, you will notice that you will only deal with potential energy due to gravity in this course. If any other source of potential energy relavent, such as electrical potential, or some other type, that will be clearly stated in the problem statement.
Internal Energy
Definition: "molecular energy", due to molecular interactions
Use: in energy balances on closed systems
Relation:
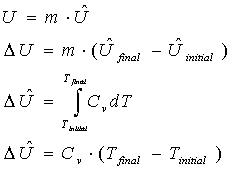
-
CV = the heat capacity at constant volume.
Here, there are two equations to find the specific internal energy using a term with a hat over it (U-hat); the third equation is used if the heat capacity is a function of temperature (often for gases). The last equation is for a constant heat capacity, (often used for liquids and solids). Each of these equations involves the use of specific properties (those with hats over them, meaning that they are "per mol" or "per mass". Once we find the specific internal energy change with the third or fourth equations, we can find the internal energy change by multiplying it by the amount of substance undergoing that change. Additionally, we can find the rate of internal energy change (DU-dot) by multiplying by the mass or molar flow rate.
It is assumed that U-hat has units of Energy/mass in the above equations, when the first formulas could be used. If U-hat has units of Energy/mole, of course, we would multiply the specific internal energy by moles (and not mass) to get the internal energy (using, for example, U = n * U-hat). It may be more correct to write U = (n or m) * U-hat. The key to getting this write is to pay attention to the units of the specific property. This of course applies to all specific properties (U-hat, H-hat, V-hat, etc.) and
is probably a point that you are already familiar with.
Example: Temperature Change of a SolidImagine a molecule, say a water molecule, is stuck in a lattice (an ice cube). Here, it is able to vibrate a little, but it is fixed in position with respect to its neighboring water molecules. If we neither increase nor decrease the temperature of the freezer (no temperature change) its internal energy ("molecular energy") doesn't change; it simply continues to vibrate to the same extent. INow, if we make the system colder, thes molecule vibrates less, and there is a change in internal energy (a decrease). If we take the ice cube out of the freezer and place it where it is warmer, the molecule vibrates more (gaining energy, an increase in internal energy). If it melts, it further gains internal energy. In this example there is a temperature change to accompany the molecule's change in internal energy.
Note: Changes in internal energy occur with processes that change its molecular energy. Thus, internal energy changes occur with a change in temperature (effects a molecule's motion), a chemical reaction (breaking and making bonds), a change of state (change in intermolecular energy), or a substantial pressure change(under high pressures, our H20 molecule in our ice cube vibrates less).
Enthalpy
Definition: "molecular energy", due to molecular interactions
Use: in energy balances on open systems
Relation:
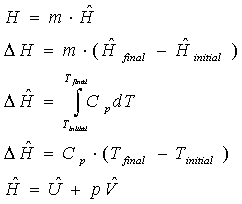
- Cp = the heat capacity at constant pressure.
Here, the usage of the first -> fourth equations is analogous to scenarios for those equations for internal energy already explained above. However, the last equations describe the relationship between enthalpy and internal energy. Similar discussions given above in the internal energy section about flow rates and specific properties are applicable here.
Example: Enthalpy can be thought of as the "molecular energy," or the internal energy for moving fluids. Thus, if a flow of water in a pipe experiences a change in temperature, it is accompanied by a change in enthalpy.
Note: A system experiences a change in enthalpy if there is a change in temperature, a substantial change in pressure, a chemical reaction, or if there is a change in state.
Note: Additionally, there is a relationship between the heat capacity at constant volume and the heat capacity at constant pressure, this relation is deferred until later.
|