(chapter 4, A Reaction) Example:
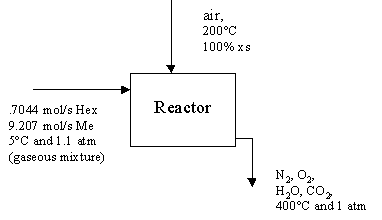
.7044 mol/s Hexane and 9.287 mol/s Methane and 100 % excess air are fed into a reactor to produce only CO2 and H2O as products of the reaction. Determine all inlet and outlet flow rates, and the extents of reaction.
Flow Diagram:
Our Reactions
Here are the reactions that occur in our reactor.
CH4 + O2 ® CO2 + H2O
1CH4 + 2O2 ® 1CO2 + 2H2O, and call this reaction #2
And, the useful formulas are,
And, rearranging this to show that we can calculate x if we just know how many moles of any of the components that reacted (because we would already know vi for that species from the reaction),
and finally, our percent excess formula,
where, "stoich" referes to the number of moles that react.
Here now, we need not write out the first equation given for each component, because we can calculate the extents of reaction with the second equation, but we proceed to write out the first equation for each component, for illustrative purposes. Here, out referes to the outlet stream, and in referes to the respective inlet stream.
ndotout,Me = 9.287mol/s + (0)*x1 + (-1)*x2 = 0
ndotout,O2 = ndotin,O2 + (-9.5)*x1 + (-2)*x2
ndotout,H2O = 0 + (+7)*x1 + (+2)*x2
ndotout,CO2 = 0 + (+6)*x1 + (+1)*x2 = 0
ndotout,N2 = ndotin,N2 + (0) + (0) = 0
x2 = 9.287 mol/s
ndotout,O2 = ndotin,O2 + -25.26 mol/s
ndotout,H2O = 23.50 mol/s
ndotout,CO2 = 13.51 mol/s
ndotout,N2 = ndotin,N2
Now, we can go no farther, and we find that we can still use the %xs relation, so, given air in 100% xs, this gives,
or,
and since ndotO2 stoich = 9.5x1 + 2x2, and we have found the extents of reaction, thus we get ndotO2 stoich = 25.26 mol/s, now, if we plug this into our equation, we get ndotO2 fed = 50.52 mol/s.
Now, we can readily get the fed N2, and we get .79/.21 * 50.52 mol/s = 189.0 mol/s.
Now, going back to our O2 balance, with the feed O2 now determined for the %xs relation, we can now sub in this value to get the O2 in the outlet, to get, ndotout,O2 = 25.26 mol/s. Now, all flows have been determined as have both extents of reaction.
x2 = 9.287 mol/s
| Hexane | Methane | Oxygen | Nitrogen | CO2 | Water | |
|---|---|---|---|---|---|---|
| Inlets | .7044 | 9.207 | 50.52 | 189.0 | X | X |
| Outlets | 9.992 | .7044 | 25.26 | 189.0 | 13.51 | 23.50 |
All flowrates in mol/s.
