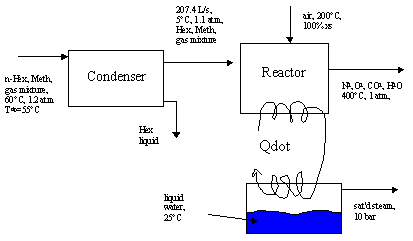
Problem Statement:
A gas stream consisting of n-hexane in methane is fed to a condenser at 60°C and 1.2 atm. The dew point of the gas (considering hexane as the only condensable component) is 55°C. The gas is cooled to 5°C in the condenser, recovering pure hexane as a liquid. The effluent gas leaves the condenser saturate with hexane at 5°C and 1.1 atm and is fed to a boiler furnace at a rate of 207.4 L/s, where it is burned with 100% excess ari that enters the furnace at 200°C. The stack gas emerges at 400°C and 1 atm and contains no carbon monoxide or unburned hydrocarbons. The heat transferred from the furnace is used to generate saturated steam at 10 bar from liquid water at 25°C.
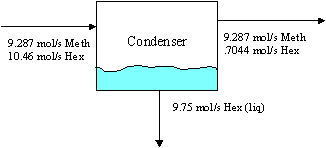
a) Calculate the mole fracions of hexane in the condenser feed and product gas streanms and the rate of hexane condensation.
b) Calculate the rate at which heat must be transferred from the condenser and the rate of generation of steam in the boiler (kg/s).
Initial Sketch:
We use the ideal gas law and vapor-liquid equilibrium relations (chapter 6, Raoult's Law) for the condenser. This analysis answers part a), and readily gives (to see the condenser balance, click here):
Where, because air is in excess, there is oxygen and nitrogen in the outlet streams, and the problem statement tells us no hydrocarbons are into the product stream, and furthermore that only CO2 (and of course water) are the only combustion products.
For an energy balance around this equimpment, we need to know how to deal with the enthalpy changes of a temperature change and a reaction. Again, always for DH of reactions (as with phase changes) we need to get to STP. For us, it results in the following energy balance calculation path (considering that the gases are ideal, or that they are all ~1 atm, the standard pressure, either way, we don't need to consider the enthalpy change due to pressure in our calculation for the energy balance around the reactor).

This will give us the heat rate that is transferred to the boiler. Here are the reactions that occur in our reactor.
CH4 + O2 ® CO2 + H2O
1CH4 + 2O2 ® 1CO2 + 2H2O
Now, lets begin the calculation process to get the DH around the reactor. Let's follow this algorithm.
1)calculate the DH for temperature changes, for the inlets going to STP, and for the outlets going from STP.
2)determine DHhatrxn°, for both reactions.
3)determine DHrxn°, for both reactions, by first obtaining x's from material balances.
4)add all the inlet, reaction, and outlet DH's.
1)The heat capacity for each gas can be looked up in appendix B2, where it is given as a polynominal. This should be programmed into your calculator (Click here to see my program for my ti-89 and a program for the ti-86).
So, we apply the following formula,
Now, for the other inlet stream, we have Tinitial = (5 + 273)K, and Tfinal = (298)K, for both Methane and Hexane, so, after looking up the parameters and taking the integral, we get, DHhatMe =
And, for the product stream, we go from Tinitial = 298K to Tfinal698K, looking up a,b,c, and d for each outlet component, evaluating the integral and applying these limits gives, DHhatO2 = +12.522 kJ/mol, DHhatN2 = +11.66 kJ/mol, DHhatCO2 = +18.937 kJ/mol, DHhatH2O = +14.33 kJ/mol
, now, we need DH's, so multiply each but their molar flow rate,
inlets:
DHO2 = -5.727 kJ/mol * ? DHN2 = -5.344 kJ/mol * ? DHMe = +.7024 kJ/mol * 9.287 mol/s= 6.523 kJ/s DHHex = +4.732 kJ/mol * .7044 mol/s = 3.333 kJ/mol outlets:Here now, we can see that we don't have all the flow rates, this must come from somewhere else, we have not done the material balances, so perhaps we can get these unknown inlet and outlet flow rates for a material balance. This is indeed the case, so we will move to step 2, understanding that we will have to pick up these last few calculations after the material balances, which are done in step 3, are performed.
DHO2 = +13.522 kJ/mol * ? DHN2 = +11.66 kJ/mol * ? DHCO2 = +18.937 kJ/mol * ? DHH2O = +14.33 kJ/mol * ?
2)
It turns out, that since we are performing combustion reactions, that only yeild CO2 and H2O, we can use the DHhatcomb’ values in appendix B1 for methane and for hexane, these are the DHhatrxn’ for the entire combustion reaction. But, to ilustrate the determination of DHhatrxn’ from heats of formation, which is the usual calculation process, it is done here for the methane reaction. We will see that after applying our method, it matches the value already given for the heat of combustion.
our formula is:
This is our process for finding DHhat’rxn using heats of formation, but as we can see, for the special case of combustion reactions that only yeild CO2, we can use the heats of combution tabulate values in B1. We will resort to this for the Hexane reaction, so, from appendix B1, we get:
3)First, here, we will write the DH’rxn,i equation for both reactions.
DH°rxn2 = x 2*DH°hatrxn2
Now we will determine the extents of reaction needed here and the molar flow rates of the components in the inlet and outlet streams that we need to complete the part 1 calculation.
To see the material balances around the reactor, click here. Here are the results of performing the material balances on the reactor:
x2 = 9.287 mol/s
| Hexane | Methane | Oxygen | Nitrogen | CO2 | Water | |
|---|---|---|---|---|---|---|
| Inlets | .7044 | 9.207 | 50.52 | 189.0 | X | X |
| Outlets | 9.992 | .7044 | 25.26 | 189.0 | 13.51 | 23.50 |
All flowrates in mol/s.
The first reaction cooresponds to the reaction of hexane, and the second cooresponds to the reaction of methane, and having found the extents of reactions and the specific standard enthalpy changes of the combustion reaction, we get,
DH°rxn,Me = 9.287 mol/s * (-)890.33 kJ/mol = (-)8268.5 kJ/s
Now, we go back to finish the calculations in part 1.
1)The calculations for O2, N2, CO2, and H2O can now be made.
inlets: DHO2 = -5.727 kJ/mol * 50.52 mol/s = (-)289.3 kJ/s DHN2 = -5.344 kJ/mol * 189.0 mol/s = (-)1010.0 kJ/s DHMe = +.7024 kJ/mol * 9.287 mol/s = 6.523 kJ/s DHHex = +4.732 kJ/mol * .7044 mol/s = 3.333 kJ/mol outlets:
DHO2 = +13.522 kJ/mol * 25.26 mol/s = 341.6 kJ/s DHN2 = +11.66 kJ/mol * 189.0 mol/s = 2203.7 kJ/s DHCO2 = +18.937 kJ/mol * 13.52 mol/s = 256.0 kJ/s DHH2O = +14.33 kJ/mol * 23.50 mol/s = 336.7 kJ/s
4)Now, we add up the enthalpy changes for the temperature changes of the inlets and outlets, along with the enthalpy changes of the two reactions, the get the entire enthalpy change around the reactor.
This says that our reactor produces 9374.7 kJ/s to be transferred to the boiler. Now we are finished with the reactor analysis. To answer part b) of the question, the first part, the heat the must be transferred from the condenser. This requires a heat balance around the condenser. This is the heat transfer for a phase change, which is a chapter 8 topic. We have not done this yet, to see the energy balance on the condenser, click here.
For the second part of question b, for the rate of steam generation in the boiler, this is another chapter 8 problem, for we use the energy balance to determine the rate of steam generation. To see this, click here. This completes the problem.
