Concentration
Upon studying this section, you should be familiar with the following:
- Units of mass and a mole concentrations
- Either a mass or mole concentration divided by a unit of volume
- The mathematical relationships for molarity, mass fractions, mole fractions, ppm, and ppb
- How to distinguish between mass and mole fraction notation
- x for solids and liquids, y for gases
- Each x or y can be for mole or mass fractions
|
Explanation: Concentration tells us how much of one component (in a mixture) we have relative the mixture (or another component in the mixture). That is, for a mixture of sugar and water, a concentration can specify how much water we have relative to the solution (water and sugar), or how much water we have relative to the other component (sugar). These concentrations can be expressed using units of amount (mols, molecules, ...), mass (kg, lb, ...), and volume (m3, L, ...). The following are some ways to express concentration, where the numerator signifies the solute and the denominator signifies the solution. Mass Concentration: g / cm3, lbm / ft3, kg / in3 Molar Concentration: kmol / m3, lb-mol / ft3, g-mol / L The last molar concentration listed, g-mol/L is the Molarity of the solute in the mixture. Thus, the definition of molarity follows: Molarity of component A, MA = g-molA / Ltotal Mass and Mole Fractions Mass and mole fractions will be used frequently in material and energy balance problems. The notation used throghout the majority of this course and in this online textbook differs from the notation introduced in chapter 3. Here, x designates a liquid or solid mass or mole fraction, and y designates mass or mole fraction for a gas. Here are the definitions for mass and mole fractions: Equation 1: xA = mA / mtotal Equation 2: xA = nA / ntotal Equation 3: yA = mA / mtotal Equation 4: yA = nA / ntotal xA, yA - mass or mole fraction of component A.
"x" is used for liquids and solids, thus the first two equations are the mass and mole fractions for liquids and solids. Accordingly, the last two equations refer to gases. As you can see, "xA" can donote several different things. For example, xH2O can refer to either the mass or mole fraction of water or ice in a mixture. It will be up to the problem solver to keep track of how "xA" is defined in working a problem. PPM and PPB Related to the mass and mole fractions are the definitions for parts per million (ppm) and the parts per billion (ppb). Here, we simply multiply the mass or mole fraction by 106 for ppm, and by 109 for ppb. Parts Per Million (ppm) = xA*106 or yA*106 Parts Per Billion (ppb) = xA*109 or yA*109 note #1: The mole and mass fractions must always be unitless, so the mole/mass of the component (numerator) and the total mole/mass (denominator) must both have the same units. note #2: Mass ratios are usually used for liquids and solids, while mole rations are usually used for gases. Example 1 A mixture of gases has the following composition by mass:
Example 2 Air is approximately 79 mole% N2 and 21 mole% O2. Calculate the average molecular weight of air by using the following formula:
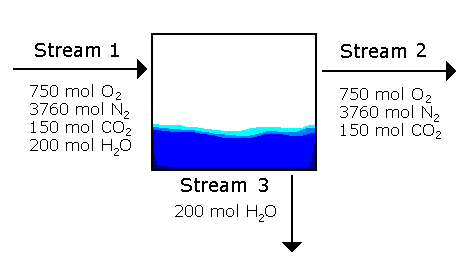
 Example 3 A material balance problem from chapter 4 gives us the following information about streams going in and out of a condenser. Determine the mole fractions of each stream.
|

The University of Arizona. All copyrights © reserved.